이미지 확대

[Courtesy of Kolon TissueGene]
SEOUL -- The U.S subsidiary of Kolon Life Science, a biosimilar arm of South Korea's Kolon Group, has resumed clinical trials at American hospitals to verify the efficacy of the world's first cell-mediated gene therapy for degenerative arthritis by recruiting some 1,020 patients before domestic stock market regulators take action on delisting over an alleged research mistake.
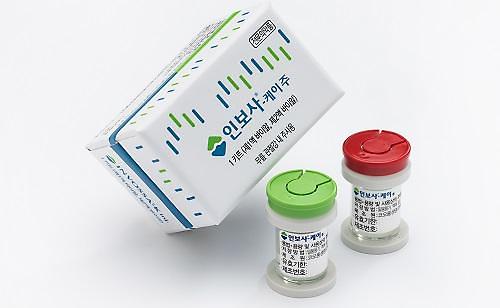
Invossa code-named "TG-C" is a novel cell and gene therapy targeting knee osteoarthritis through a single intra-articular injection. Patient recruitment for clinical trials was suspended due to product identity concerns in 2019 after Kolon TissueGene found during U.S. clinical trials that a key ingredient was different from that described in the data submitted at the time of permission in South Korea.
Kolon TissueGene said on December 28 that it has resumed administrating TG-C for phase 3 clinical trials at 80 U.S. medical institutions, starting with Source Healthcare Hospital in Santa Monica, California. The company aims to complete clinical trials in 2023.
Kolon TissueGene CEO Sung Han expressed guarded optimism, saying that phase 3 clinical trials would produce good results as scientific data obtained through previous trials were reliable. "We will successfully complete the clinical trial so that we can establish ourselves as a game-changer not only in the U.S. but also in the global osteoarthritis market."
The company will assess pain and function endpoints, as well as MRI, X-Ray and liquid biomarkers. In addition to significant pain and function improvements, clinical trials are designed to achieve the designation of a disease-modifying osteoarthritis drug (DMOAD) that would inhibit or even reverse the progression of osteoarthritis.
Kolon TissueGene avoided stock market delisting at home by receiving a one-year grace period from Korea Exchange after the U.S. Food and Drug Administration sent a letter in April 2020 that a temporary ban on clinical trials has been lifted. However, patient enrollment had been delayed due to a COVID-19 pandemic. The resumption of clinical trials at U.S. hospitals came after the grace period expired on December 17, 2021.
Invossa has been administered to more than 3,500 people for 11 years at home, but Kolong Life officials claimed to have discovered no serious side effects. Initially, Kolon Life argued that there was no cover-up or fabrication of research data, but the Ministry of Food and Drug Safety, a public health agency in South Korea, revoked the domestic license for Invossa for submitting false data about the research of Invossa to win state approval.
Previous clinical trials have demonstrated pain relief and increased mobility, as well as indicators towards the decreased progression of knee osteoarthritis and improvements in joint structure, Kolon TissueGene said, adding that the allogeneic drug could provide an alternative to traditional treatment and surgery, or delay the progression of knee osteoarthritis to minimize the need for multiple surgical interventions.
Copyright ⓒ Aju Press All rights reserved.

View more comments