[Courtesy of Sugentech]
To take the upper hand in the new market, Sugentech proposed the supply of neutralizing antibody test kits in partnership with vaccine developers, saying the development of vaccines would lead to a surge in the demand for kits to test whether neutralized antibodies have been created. A neutralizing antibody defends a cell from a pathogen or infectious particle by neutralizing any effect it has biologically.
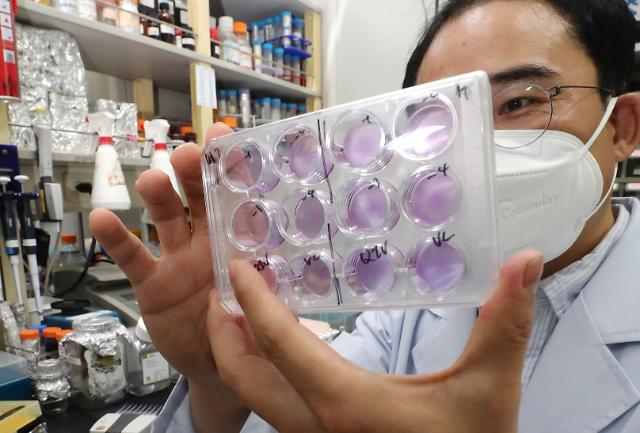
"When vaccine development becomes visible, I expect an increase in sales of neutralizing antibody test kits to vaccine development companies and specialized testing centers," Sugentech CEO Sohn Mi-jin said in an interview with Aju Business Daily. Sugentech has commercialized a variety of diagnostic medical devices including a rapid test kit called "IVnAT" that can examine the immune status of people carrying COVID-19 antibodies in two hours.
Sugentech has released or is developing products in three areas: laboratory diagnosis, clinic diagnosis, and self-diagnosis to prepare for new infectious diseases. The company's antibody test device is undergoing the so-called Laboratory Developed Test (LDT) at Avellino Lab USA.
Sugentech has prepared for a post-pandemic era by developing a mobile app that provides infection prediction, prevention and diagnosis services for new infectious diseases. The company aims to support the work of quarantine authorities with antibody diagnosis through a mobile app and allow them to check the confirmed trend of each region after the results of diagnosis is aggregated into the central server.
Currently, many test kits are used for on-site diagnosis. Sohn said that prospects for self-testing kits are bright when regulations are eased. "The same goes for other infectious diseases," she said. "Unlike nine years ago, the treatment has become customized. Prediction, diagnosis and prevention have become important. What we do is more like an analysis test, and we will take a bigger part in the bio-industry in the future."
Sohn said that COVID-19 needs a diagnostic kit for treatment or vaccine prescription because the disease itself does not end just because a cure or vaccine is developed. "In the case of influenza, the development of Tamiflu, a treatment drug, required diagnosis that distinguishes it from ordinary colds to prescribe the treatment, and thus opened a market for rapid antigen test kits."
Sugentech recorded operating losses at the time of stock market listing in May 2019. However, it has seen rapid growth this year thanks to a COVID-19 pandemic. Data from the state-run Korea Health Industry Development Institute showed that exports of South Korea's medical and health-related products including biosimilars, diagnostic kits and disinfectants rose 26.7 percent on-year to $9.6 billion in the first half of this year.
In October, Sugentech's new kit capable of screening COVID-19 and flu simultaneously won state approval in South Korea, paving the way for exports. "We will target the European market, starting with Spain and Germany," Sohn said, referring to a deal in early November to export some 200,000 rapid antigen kits to Germany-based DiaSys Diagnostic Systems.
Sohn said the deal with DiaSys would help Sugentech boost sales in Europe. Her strategy is to provide solutions tailored to the situation and quarantine policies of each country. Sohn thinks that there is more demand for antibody or antigen diagnostic kits than the molecular diagnosis in developing countries, while antibody diagnostic kits target the progress of treatment and post-care markets in the U.S. and Europe.
(This story was based on an interview by Aju Business Daily reporter Kim Tae-rim)