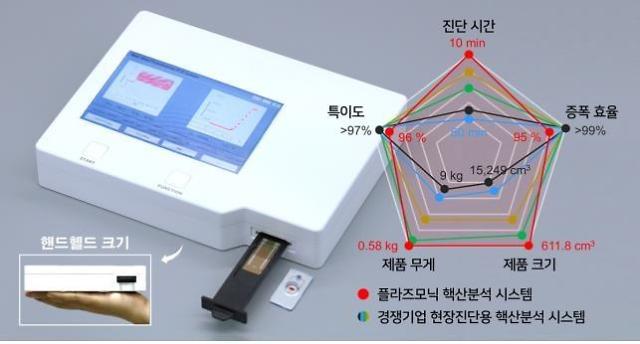
[Courtesy Yangpyeong County]
SEOUL -- In vitro diagnostic apparatus companies in South Korea have challenged an obstinate attitude taken by medical professionals in sorting out those infected with a new coronavirus, insisting it's time to introduce rapid tests using antigen or antibodies together with a traditional method.
The Korea Centers for Disease Control and Prevention (KCDC), a state health body involved in a national campaign to contain an epidemic, has adopted a real-time polymerase chain reaction (RT-PCR) method that amplifies a specific DNA sample. It takes more than six hours on average and requires trained personnel and specialized facilities.
An association of diagnostic apparatus makers said in a statement that a test examining initial antibodies produced in the body is quick, easy and cost-efficient. "When considering the characteristics of COVID-19, the accuracy should be increased by combining two tests: RT-PCR and antibody tests," the association said.
Professional medical groups have been negative, saying it's not necessary to introduce rapid immunological screening such as antibody tests. "Our country has already established a large-scale genetic testing system that enables more than 15,000 tests a day and takes six hours to secure exact results," they said in a joint statement on March 17.
"The accuracy of antigen and antibody tests is significantly lower than genetic testing. It is very dangerous to introduce inaccurate rapid tests at a time when COVID-19 is spreading worldwide," the professionals said.
Health officials have been annoyed by asymptomatic infections and the dynamic nature of COVID-19. KCDC director Jeong Eun-kyeong has attributed a high rate of asymptomatic infections to the early and extensive screening of suspected cases. "It's not clear yet whether asymptomatic patients are contagious. So far, there has been no objective evidence regarding asymptomatic transmission," she said.
On March 11, a research team at the Institute for Basic Science (IBS) said it has developed a laboratory-safe, low-cost, high-sensitivity protocol for the early detection of COVID-19 that would be helpful for the first-hand screening of asymptomatic virus carriers. However, the research team cautioned there should be more examinations for clinical use.
Two days later, Philosys Healthcare, a biomedical and medical healthcare producer in South Korea, said it has clinched a deal from TAHA Life Science, a German biotechnology company, and GNP Diagnostic of Greece, to provide new testing kits capable of diagnosing infection within 20 minutes by using saliva.
Philosys said its testing kit called Gmate COVID-19 is useful for drive-thru and mobile clinics because the process of collecting samples is simple and it takes little time to diagnose infection. The kit has yet to secure approval from health authorities.