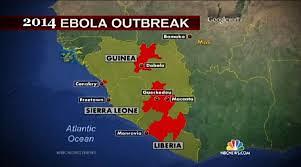
The Ebola epidemic in West Africa has killed nearly 1,000 people and prompted the World Health Organization to declare an international health emergency.
No Ebola drugs or vaccines have entered mid-stage human trials, let alone been approved. The most advanced has been tested only in monkeys and a handful of humans.
Vaccines
◆Glaxosmithkline Plc. (GSK)
GSK is co-developing a vaccine with the U.S. National Institute of Allergy and Infectious Diseases (NIAID), which has shown promising results in primates.
The vaccine is due to enter early-stage human trials, pending U.S. Food and Drug Administration approval. GSK has said human testing should start this year. NIAID expects trials to begin "as early as fall 2014."
Even if it is fast-tracked and works as well as hoped, the new vaccine could not be ready for widespread deployment before 2015.
◆Johnson & Johnson
The company's Crucell unit is working with the U.S. NIAID to develop a vaccine, which should enter early-stage clinical testing in late 2015 or early 2016. The Crucell vaccine is designed to give additional protection against Marburg, another severe and highly fatal disease caused by a virus from the same family as Ebola.
◆Bavarian Nordic A/S
The Danish company is developing its smallpox vaccine, Imvanex, for use as an anti-filovirus injection. The drug was found to be 100 percent protective against Ebola and Marburg in non-human primates.
The U.S. government awarded the company a $17.9 million contract in 2012. The injectable form of Imvanex is likely to enter Phase I clinical trials in the United States in 2015, Maxim analyst Jason Kolbert said.
◆Profectus Biosciences Inc.
The privately owned company has tested its vaccine in monkeys, with good results. A single intramuscular injection was found to protect all of the rhesus monkeys exposed to Ebola three weeks later. The company hopes to launch a human trial within 12 months.
Treatments
◆Tekmira Pharmaceuticals Corp.
The FDA has allowed the Canadian drugmaker to resume testing a drug in people infected with Ebola, after blocking human tests last month. The FDA had halted the early-stage study following safety concerns among people taking the highest doses of the drug.
◆Biocryst Pharmaceuticals Inc.
The company's BCX4430 drug is in pre-clinical trials to treat Marburg, and analysts believe it could potentially cure Ebola.
By Ruchi Singh